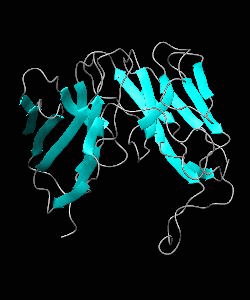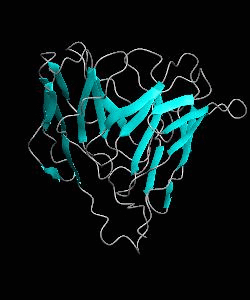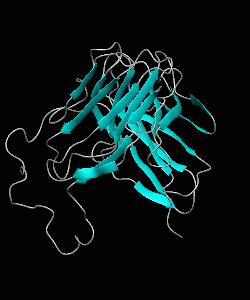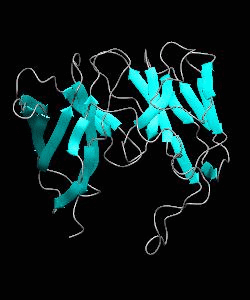
D10-scTCR
Using NMR spectroscopy, we determined the solution structure of a single-chain T-cell receptor (scTCR) derived from the major histocompatibility complex (MHC) class II-restricted D10 TCR.We infer a conserved orientation for TCR V(alpha) domains in complexes with both class I and II MHC-peptide ligands, which implies that small structural variations in V(alpha) confer MHC class preference.
1dCD2
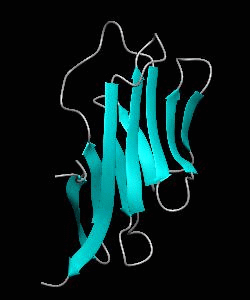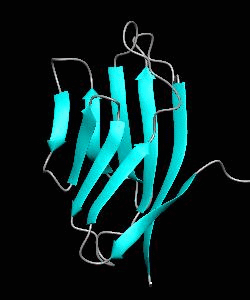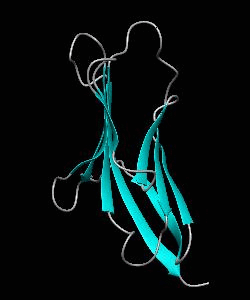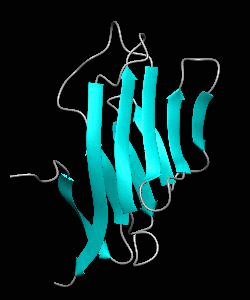
Cell surface receptors, such as CD58 and CD2, are responsible for faciliating immune responses. Here is the NMR structure of the adhesion domain of CD2, which adopts an immunoglobulin V-set fold, with the binding face to CD58 facing the front.
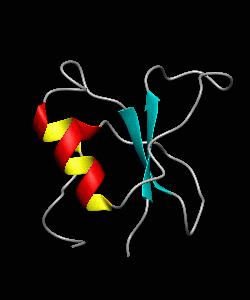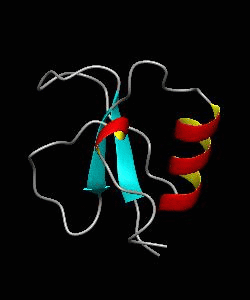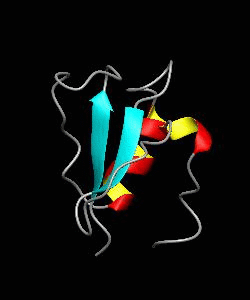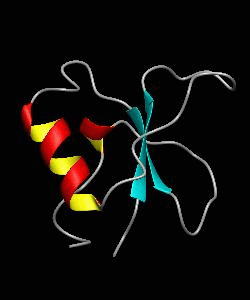
CD2BP2-GYF
T cell activation through the CD2 cell surface receptor is transmitted by proline-rich sequences within its cytoplasmic tail. Here is the solution structure of the CD2 binding domain of the novel protein CD2BP2, which we name the glycine-tyrosine-phenylalanine (GYF) domain.
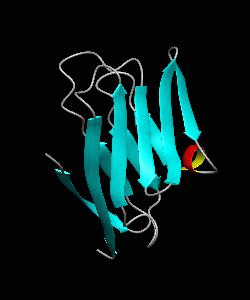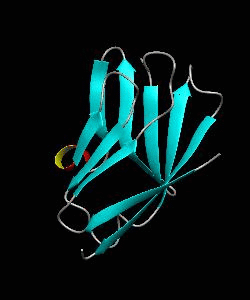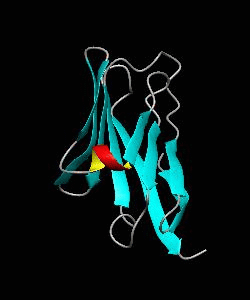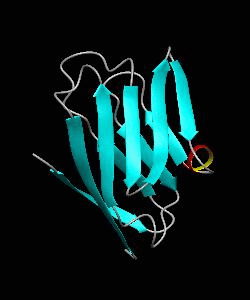
1dCD58
Cell surface receptors, such as CD58 and CD2, are responsible for faciliating immune responses. Here is the soluble 11 kDa adhesion domain extracted from the heavily glycosylated 55 kDa human CD58 ectodomain. The new structural information supports a 'hand-shake' model of CD2-CD58 interaction involving the GFCC'C" faces of both CD2 and CD58 adhesion domains.